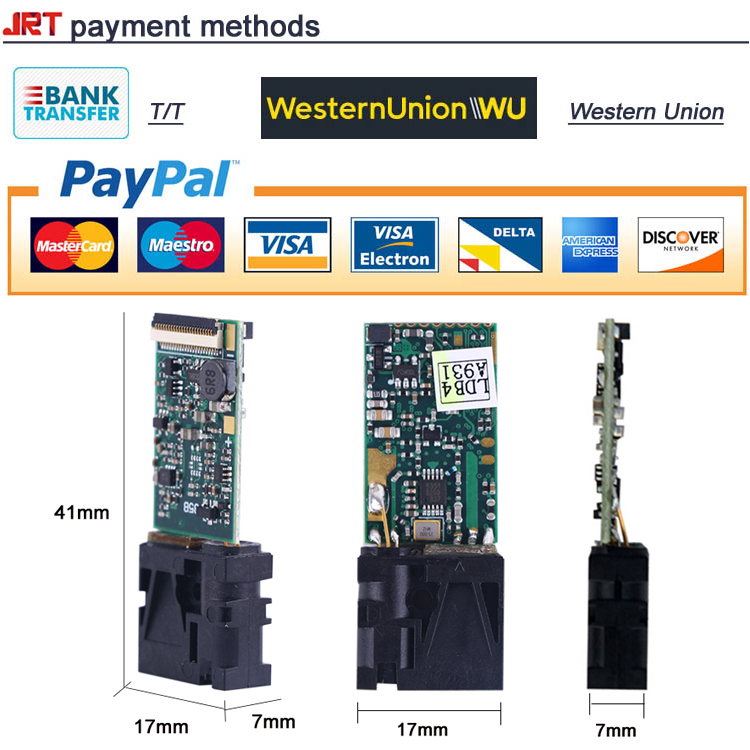
Fast Shipping Laser Distance Sensor Hi there, Fast Shipping Laser Distance Sensor,Serial Laser Distance Sensors,USB Laser Distance Sensors,RS232 Laser Distance Sensors Chengdu JRT Meter Technology Co., Ltd , https://www.cdlaserdistancesensor.com
Guo Fanli, a researcher in the pharmaceutical industry at China Investment Advisors, pointed out that China is a big country in API production, but generally low added value, although some companies have good resources and channels for export, but due to harsh foreign standards, especially the EU, the United States and other thresholds The high level of investment has led some domestic enterprises to target their developing countries. Although the thresholds and standards of these countries or regions are far lower than those of developed countries, at the same time, the profits that domestic companies can obtain are also very small.
Guo Fanli believes that at present, the world will usher in an upsurge of patent drugs. This is not only an opportunity for China's pharmaceutical industry, but also a severe challenge. With the support of policies, China's chemical drug companies must make efforts to reform the current production pattern. This requires not only many raw material companies in China to transform from simple raw material drug production to production of pharmaceutical products, but also need to upgrade the company's internal hardware and software. To meet the standards of the developed countries such as the European Union and the United States.
Zhang Yulin, research director of China Investment Consulting Co., Ltd., said that as a raw material drug producing country, over the years, China's raw material pharmaceutical companies have accumulated a lot of experience, and a batch of raw material drug companies with high-standard production capacity have emerged, such as Huahai Pharmaceutical. It is the first company that has passed the US FDA certification for its preparations. However, in the aspect of independent intellectual property rights, China has not yet achieved the “ice-breaking journey†so far, and building products with independent intellectual property rights is the top priority for the future development of the pharmaceutical industry. .
The “2010-2015 China Pharmaceutical Industry Investment Analysis and Prospect Forecast Report†issued by China Investment Advisors pointed out that in the first half of 2011, China will issue a special plan for the “quality assurance and upgrading of pharmaceutical manufacturing†program. The main content of the plan is that national finance will pass in the future. The way of subsidy encourages the development of a batch of characteristic raw material medicine companies, and supports the raw material pharmaceutical preparation enterprises with development prospects to pass the relevant certifications of European and American markets as soon as possible, and this amount is conservatively estimated to reach billions.
We creat a new payment method online for you, so that all of our customers, and you can get the samples quickly. With the competitive prices online, it is easy to operate and finish your sample orders of JRT Laser Distance Sensor. It's really amazing, right? Let's go!
Greetings
In 2010, the export value of chemical preparations in China increased by 30%
Business Club February 17 In 2010, China's chemical preparation industry came out of the shadow of the financial crisis, and domestic chemical product manufacturers' exports have made relatively good progress. Due to the stimulation of export tax rebates from 13% to 15%, the annual export value of the domestic chemical preparation industry reached 1.5 billion U.S. dollars, a year-on-year increase of 30%, and achieved remarkable results.