Gentian violet is an antiseptic dye used to treat fungal infections of the skin (e.g.,ringworm, athlete's foot). It also has weak antibacterial effects and may be used on minor cuts and scrapes to prevent infection. OTHER USES: This section contains uses of this drug that are not listed in the approved professional labeling for the drug but that may be prescribed by your health care professional. Use this drug for a condition that is listed in this section only if it has been so prescribed by your health care professional. Your doctor may also prescribe this medication for fungal infections of the mouth(thrush).  Side Effects Redness, swelling, or irritation at the application site may occur. If any of these effects persist or worsen, contact your doctor or pharmacist promptly. If your doctor has directed you to use this medication, remember that he or she has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects. Stop using this medication and tell your doctor if you have new signs of a skin infection (heat, tenderness, pus). Stop using this medication and tell your doctor right away if this rare but serious side effect occurs: skin sores. Skin sores are more likely to develop when the medication is applied to skin folds (e.g., between the toes, beneath the breasts). Be careful to apply only a small amount to skin fold areas, and allow to dry completely before putting on shoes or clothing. A very serious allergic reaction to this product is rare. However, seek immediate medical attention if you notice any of the following symptoms of a serious allergic reaction: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness,trouble breathing. This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.  Precautions Before using gentian violet, tell your doctor or pharmacist if you are allergic to it; or to other dyes; or if you have any otherallergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details. If you have a certain metabolic disease (porphyria), consult your doctor or pharmacist before using this product. Tell your doctor if you are pregnant before using this medication. It is unlikely that this product passes into breast milk. Consult your doctor before breast-feeding.  Interactions If you are using this medication under your doctor's direction, your doctor or pharmacist may already be aware of any possibledrug interactions and may be monitoring you for them. Do not start, stop, or change the dosage of any medicine before checking with your doctor or pharmacist first. Before using this product, tell your doctor or pharmacist of all prescription and nonprescription/herbal products you may use. Keep a list of all your medications with you, and share the list with your doctor and pharmacist.  Overdose This medicine may be harmful if swallowed. If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center. Symptoms of overdose may include:nausea, vomiting, diarrhea.  Shelf time: Â
This classification summarizes the Chlorella Powder produced by our own factory in northwestern of China .
We have advanced equipment and strict quality control system to ensure the quality and production.
The products under the classification are:
1. Chlorella Powder .
Various parameter specifications of our product:
Naturland Certified ; CERES certified .
About Company Chlorella Powder Chlorella Powder,Organic Pure Chlorella Powder,Natural Organic Chlorella Powder,Chlorella Vulgaris Powder YANCHI YI JIAN BIOLOGICAL PROJECT CO.,LTD , http://www.spirulina-yj.com
Product name:Â
STORAGE INSTRUCTIONS:
Store below 25°C in a closed bottle.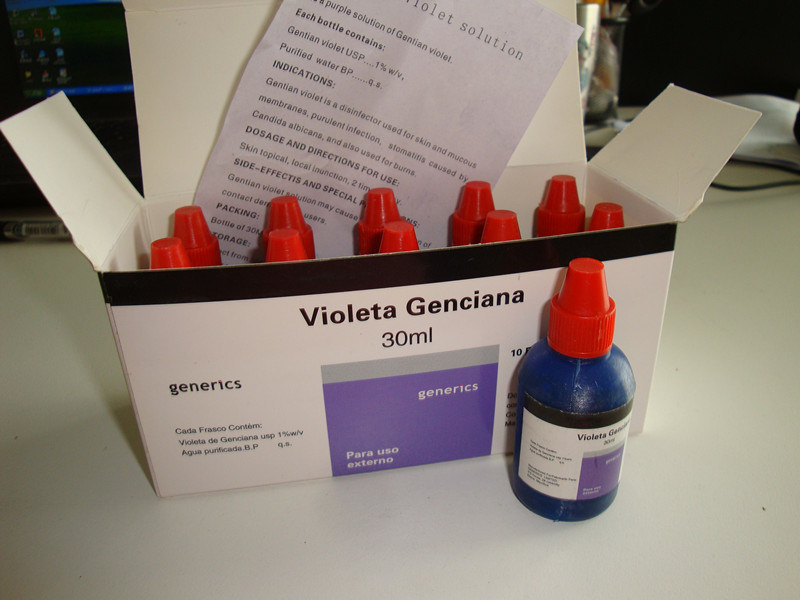
Â
Â

EU & NOP standard ; Kosher & Halal Available .
Low heavy metals & Micro Contents , Low & Stable PAH4 Level ,
PAH4 value is less than 10 ppb .Low microorganismsNon-Irradiation ,
Non GMO , Gluten Free , Allergen Free , Pesticides Free .
Own Factory : Manufacture in northwest of China . Legitimacy , Regularity , Cultural .
Own Lab : Quality control and Product development . Strictly , Creativity , Responsibility .

Yanchi County Yijian Biotechnol Co.,Ltd
was founded in Dec 2012 ,
by Mr. Dezhi Zhang ,
the legal representative of the company .
Company registered capital is 10 million RMB .
The main business sectors are culture , processing , internal sales , import and export trade of Organic Spirulina and Organic Chlorella products .
Yijian is known globally as one of the major suppliers of microalgae products across the world .
Annual production rate is 600 Mt .
Average annual sales income is around 5 million dollar .
External Medication Gentian Violet Solution, Gentian Violet for Thrush / Mouth Sores Medical Drugs
Model NO.: UNPGEN
Pharmaceutical Technology: Chemical Synthesis
Drug Reg./Approval No.: H20031108
Drug Ad Approval No.: No
Trademark: HU
Transport Package: Plastic Bottle-Carton
Specification: 30ml/bottle
Origin: China
HS Code: 3004909099
GMP Certified High Quality USP Standard Gentian Violet Solution for mouth 1% W/V 30ml/bottle, 10bottles/carton
Gentian Violet Solution 1% W/V
Â
Uses
3 yearsÂ